Loading... Please wait...
Loading... Please wait...- Home
- Disease Area
- Infectious Disease
- Zanamivir | Neuraminidase inhibitor
Product Description
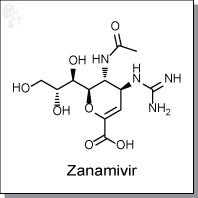
Zanamivir is a dihydropyran-based, sialic acid analog inhibitor of the viral enzyme neuraminidase. Zanamivir's mode of action is blockage of sialic acid cleavage, thereby preventing further virus distribution. For type A viruses zanamivir has IC50s of 0.35 nM for the H1N1 subtype, and 1.1 nM for the H3N2 subtype. [1]
In plaque reduction in vitro assays, zanamivir inhibits a wide range of Type A and Type B influenza viruses with IC50s of plaque formation ranging from 5 to 14 nM in laboratory-passaged strains, and 20 to 1600 nM for clinical isolates. In human respiratory epithelial cells, EC90s of virus yields were determined to be less than 0.01 ug/mL at 24h for both H1N1 and H3N2 subtypes. [2]
Technical information:
| Chemical Formula: | C12H20N4O7 | |
| CAS #: | 139110-80-8 | |
| Molecular Weight: | 332.31 | |
| Purity: > 98% | ||
| Appearance: | White | |
| Chemical Name: | (2R,3R,4S)-3-acetamido-4-guanidino-2-((1R,2R)-1,2,3-trihydroxypropyl)-3,4-dihydro-2H-pyran-6-carboxylic acid | |
| Solubility: | Up to 50 mM in DMSO | |
| Synonyms: | Relenza, Zanamavir, Sialic acid derivative |
Shipping Condition: The product is shipped in a glass vial at ambient temperature.
Storage condition: For longer shelf life, store solid powder at 4oC desiccated, or store DMSO solution at -20oC.
Reference:
| 1. | Colman et al., Zanamivir: an influenza neuraminidase inhibitor. Expert Rev. Anti. Infect. Ther. 2005, 3(2), 191-199. |
| 2. | Elliott et al., Zanamivir: from drug design to the clinic. Phil. Trans. R. Soc. Lond. 2001, 356, 1885-1893. |
| 3. | McNicholl et al., Neuraminidase Inhibitors: Zanamivir and Oseltamivir. Annals Pharmacother. 2001, 35, 57-70. |
Other Information:
Product Specification (pdf)
MSDS (pdf)
Certificate of Analysis is available upon request.