Loading... Please wait...
Loading... Please wait...- Home
- Cellular Mechanism
- Epigenetics
- Histone Deacetylases (HDAC)
- TMP195 | Class IIa HDAC inhibitor
Product Description
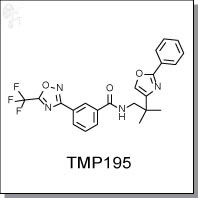
TMP195 is the most potent and selective class IIa HDAC inhibitor identified to date, with IC50s of 59 nM, 60 nM, 26 nM and 15 nM for HDAC4, HDAC5, HDAC7 and HDAC9 respectively. It has over 100-fold selectivity targeting other HDACs (IC50s>10 µM). TMP195 has an unprecedented zinc-binding group, trifluoromethyloxadiazole (TFMO), which circumvents the selectivity and pharmacologic liabilities of hydroxamates. (1)
TMP195 alters the gene expression of monocytes upon treatment of colony-stimulating factors, while showing no cytotoxicity towards T cell, B cells and monocytes. This tool compound help revealed the unique cellular activities of class IIa HDACs. (1) The discovery of TMP195 and related compounds provides an alternative design for targeting metalloenzymes than the conventional chelating metal-binding group, and suggests a therapeutic potential for class IIa HDAC inhibitors distinct in mechanism and application compared to current HDAC inhibitors (e.g. Trichostatin A, or TSA).
Technical information:
| Chemical Formula: | C23H19F3N4O3 | |
| CAS #: | N/A | |
| Molecular Weight: | 456.42 | |
| Purity: | > 98% | |
| Appearance: | White | |
| Chemical Name: | N-(2-methyl-2-(2-phenyloxazol-4-yl)propyl)-3-(5-(trifluoromethyl) -1,2,4-oxadiazol-3-yl)benzamide | |
| Solubility: | Up to 50 mM in DMSO | |
| Synonyms: | TMP195 |
Shipping Condition: The product is shipped in a glass vial at ambient temperature.
Storage condition: For longer shelf life, store solid powder at 4oC desiccated, or store DMSO solution at -20oC.
Reference:
| 1. | Lobera M, et al. Selective class IIa histone deacetylase inhibition via a nonchelating zinc-binding group. (2013) Nat Chem Biol. 9(5):319-25. Pubmed ID: 23524983 |
Other Information:
Product Specification (pdf)
MSDS (pdf)
Certificate of Analysis is available upon request.