Loading... Please wait...
Loading... Please wait...- Home
- Molecular Target
- Protease
- Ser/Thr/Cys protease
- Rivaroxaban (Xarelto) | Protease Factor Xa inhibitor
Product Description
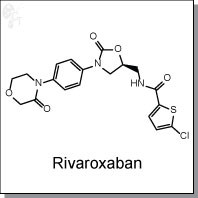
Rivaroxaban is an orally-bioavailable, oxazolidinone-based inhibitor (Ki = 0.4 nM) of factor Xa, the factor in the blood coagulation pathway leading to thrombin generation and clot formation. [1] It is approved for both deep vein thrombosis and pulmonary embolism. It is >10000-fold selective for fXa over other biologically relevant serine proteases. Rivaroxaban inhibits prothrombinase-bound (IC50 = 2.1 nM, and clot-associated factor Xa (IC50 = 75 nM) in a dose-dependent manner. [1]
In vivo, Rivaroxaban inhibits endogenous fXa more potently in human and rabbit plasma (IC50 = 21 nM) than in rat plasma (IC50 = 290 nM). [2] In human plasma, PT and aPTT were doubled at 0.23 and 0.69 uM, respectively.
Technical information:
| Chemical Formula: | C19H18ClN3O5S | |
| CAS #: | 366789-02-8 | |
| Molecular Weight: | 435.88 | |
| Purity: | >99% | |
| Appearance: | White | |
| Chemical Name: | (S)-5-chloro-N-((2-oxo-3-(4-(3-oxomorpholino)phenyl)oxazolidin-5-yl)methyl)thiophene-2-carboxamide | |
| Solubility: | Up to 100 mM in DMSO | |
| Synonyms: | Rivaroxaban, Xarelto, BAY-59-7939, BAY 59-7939, BAY59-7939 |
Shipping Condition: The product is shipped in a glass vial at ambient temperature.
Storage condition: For longer shelf life, store solid powder at 4oC desiccated, or store DMSO solution at -20oC.
Reference:
| 1. | Perzborn et al., Rivaroxaban: a new oral factor Xa inhibitor. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 376-381. Pubmed ID: 20139357 |
| 2. | Perzborn et al., In vitro and in vivo studies of the novel antithrombotic agent BAY 59-7939--an oral, direct Factor Xa inhibitor. J. Thromb. Haemost. 2005, 3(3), 514-521. Pubmed ID: 15748242 |
Other Information:
Product Specification (pdf)
MSDS (pdf)
Certificate of Analysis is available upon request.