Loading... Please wait...
Loading... Please wait...- Home
- Cellular Mechanism
- Angiogenesis
- FG-4592 (ASP1517) | HIF prolyl-hydroxylase inhibitor
- Home
- Cellular Mechanism
- Metabolism
- FG-4592 (ASP1517) | HIF prolyl-hydroxylase inhibitor
- Home
- Disease Area
- Renal Disease
- FG-4592 (ASP1517) | HIF prolyl-hydroxylase inhibitor
Product Description
FG-4592 is an orally-available isoquinoline-based inhibitor of hypoxia-inducible factor (HIF) prolyl hydroxylase for the treatment of anemia and patients with chronic kidney disease. [1] Phase 2 studies indicate that oral administration of FG-4592 three times a week increased mean hemoglobin levels in the first eight weeks, regardless of supplementation with IV or oral iron, or no iron supplementation. FG-4592 is novel in that it allows integration of red blood cell production and efficient iron incorporation simultaneously. [1]
Data from Phase 2 trials comparing with epoetin alpha indicate that treatment with FG-4592 alone results in a sustained reduction of total plasma cholesterol levels by an average of 20%, while no reduction was seen with epoetin alpha. [2, 3]
In December, 2012, Fibrogen and Astellas Pharma announced the initiation of a Phase 3 clinical development program for the treatment of anemia associated with chronic kidney disease in patients not on dialysis and on dialysis. [4]
Technical information:
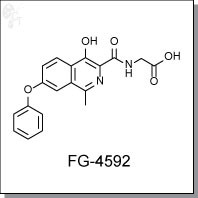
| Chemical Formula: | C19H16N2O5 | |
| CAS #: | 808118-40-3 | |
| Molecular Weight: | 352.34 | |
| Purity: | > 98% | |
| Appearance: | Yellow | |
| Chemical Name: | 2-(4-hydroxy-1-methyl-7-phenoxyisoquinoline-3-carboxamido)acetic acid | |
| Solubility: | Up to 100 mM in DMSO | |
| Synonyms: | FG-4592, FG4592, ASP1517 |
Shipping Condition: The product is shipped in a glass vial at ambient temperature.
Storage condition: For longer shelf life, store solid powder at 4oC desiccated, or store DMSO solution at -20oC.
Reference:
| 1. | "Correction of anemia without IV iron supplementation in incident dialysis patients" Fibrogen press release, Nov. 5, 2012 |
| 2. | "FG-4592 and epoetin alpha in Phase II anemia study", Datamonitor Research Store, Nov. 11, 1012. |
| 3. | "Hemoglobin correction and maintenance in end-stage renal patients" Fibrogen press release, Nov. 5, 2012 |
| 4. | Fibrogen press release, Dec. 11, 2012 (Phase 3 announcement) |
Other Information:
Product Specification (pdf)
MSDS (pdf)
Certificate of Analysis is available upon request.