Loading... Please wait...
Loading... Please wait...- Home
- Disease Area
- Infectious Disease
- Cefdinir | Antibiotic
Product Description
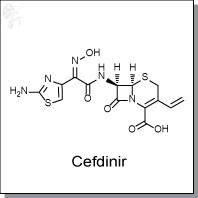
Cefdinir is an orally-available, b-lactam antibiotic that binds to penicillin-susceptible Staphylococcus aureus 2132 at an MIC of 25 nM (1) and E. Coli C11 at an MIC of 63 nM (ED50 of 1.32 mg/kg) (2) In contrast to other oral cephalosporins, cefdinir retains substantial activity against a panel of bacterial strains and is found to be extremely stable against numerous b-lactamases. Additionally, cefdinir inhibits the adherence of type-1 fimbriated E. coli to uroepithelial cells. (3)
In clinical studies, cefdinir has been shown to be superior to pencillin V and comparable to cephalexin and amoxicillin/clavulanate in the treatment of both pediatric and adult bacterial infections. (3)
Technical information:
| Chemical Formula: | C14H13N5O5S2 | |
| CAS #: | 91832-40-5 | |
| Molecular Weight: | 395.41 | |
| Purity: | > 98% | |
| Appearance: | white | |
| Chemical Name: | (6R,7R)-7-[(2Z)-2-(2-amino-1,3-thiazol-4-yl)-2-(N-hydroxyimino)acetamido]-3-ethenyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid | |
| Solubility: | Up to 100 mM in DMSO | |
| Synonyms: | Cefdinir, FK482, CI-983, and PD134393,Omnicef |
Shipping Condition: The product is shipped in a glass vial at ambient temperature.
Storage condition: For longer shelf life, store solid powder at 4oC desiccated, or store DMSO solution at -20oC.
Reference:
| 1. | Yamaguchi et al., In Vitro and In Vivo Antibacterial Activities of CS-834, a New Oral Carbapenem. Antimicrob. Agents Chemother. 1998, 42(3), 555-563 Pubmed ID: 9517932 |
| 2. | Fukuoka et al., Efficacy of CS-834 against Experimental Pneumonia Caused by Penicillin-Susceptible and -Resistant Streptococcus pneumoniae in Mice. Antimicrob Agents Chemother. 1998, 42(1), 23-27. Pubmed ID: 9449255 |
| 3. | Guay et al., Cefdinir: An Expanded-Spectrum Oral Cephalosporin. Annals Pharmacother. 2000, 34, 1469-1477. Pubmed ID: 11144705 |
Other Information:
Product Specification (pdf)
MSDS (pdf)
Certificate of Analysis is available upon request.