Loading... Please wait...
Loading... Please wait...- Home
- Disease Area
- Oncology
- BSI-201 (Iniparib) | PARP inhibitor
- Home
- Cellular Mechanism
- DNA Damage & Repair
- BSI-201 (Iniparib) | PARP inhibitor
- Home
- Cellular Mechanism
- Apoptosis
- BSI-201 (Iniparib) | PARP inhibitor
- Home
- Cellular Mechanism
- Metabolism
- DNA/RNA Synthesis
- BSI-201 (Iniparib) | PARP inhibitor
Product Description
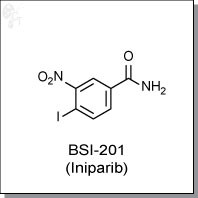
BSI-201 (Iniparib), a iodonitrobenzamide-based cytotoxic agent, was initially considered to be a PARP inhibitor based on its abillity to inactivate PARP by means of zinc ejection from the zinc finger of the enzyme. [1]
Despite its ability to kill normal and neoplastic cells at high concentrations (>40 uM), further studies revealed that BSI-201 did not selectivly kill homologous-recombination (HR)-deficient cells, sensitize cells to topoisomerase I poisons, or inhibit PARP in situ, as seen with olaparib and veliparib. [2]
Through a battery of enzymatic, cellular, and viability assays, BSI-201 was shown to nonselectively modify cysteine-containing proteins in tumor cells. It is also postulated that the formation of nonspecific adducts can alter stability, activity, and localization, thus inducing apoptosis, stress, cell-cycle perturbation, or DNA damage. [3]
Technical information:
| Chemical Formula: | C7H5IN2O3 | |
| CAS #: | 160003-66-7 | |
| Molecular Weight: | 292.03 | |
| Purity: | > 98% | |
| Appearance: | White | |
| Chemical Name: | 4-iodo-3-nitrobenzamide | |
| Solubility: | Up to 100 mM in DMSO | |
| Synonyms: | BSI-201, BSI201, BSI 201, 160003-66-7, Iniparib |
Shipping Condition: The product is shipped in a glass vial at ambient temperature.
Storage condition: For longer shelf life, store solid powder at 4oC desiccated, or store DMSO solution at -20oC.
Reference:
| 1. | Mendeleyev et al., Potential chemotherapeutic activity of 4-iodo-3-nitrobenzamide. Biochem. Pharmacol. 1995, 50(5), 705-714. Pubmed ID: 7669074 |
| 2. | Patel et al., Failure of Iniparib to inhibit poly(ADP-Ribose) polymerase in vitro. Clin. Cancer Res. 2012, 18, 1655-1662. Pubmed ID: 22291137 |
| 3. | Liu et al., Iniparib nonselectively modifies cysteine-containing proteins in tumor cells and is not a bonafide PARP inhibitor. Clin. Cancer Res. 2012, 18, 510-523. |
Other Information:
Product Specification (pdf)
MSDS (pdf)
Certificate of Analysis is available upon request.