Loading... Please wait...
Loading... Please wait...- Home
- Disease Area
- Oncology
- Abiraterone | P450 CYP17 inhibitor
- Home
- Cellular Mechanism
- Metabolism
- Abiraterone | P450 CYP17 inhibitor
Product Description
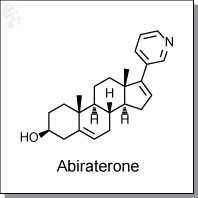
Abiraterone is a potent steroidal inhibitor against cytochrome P450 17alpha-hydroxylase-17,20-lyase (CYP17) with an IC50 at 4 nM. It was approved by the FDA in April 2011 to treat castration-resistant prostate cancer. A phase I/II clinical trial evaluating abiraterone acetate in advanced breast cancer patients are also underway. In preclinical studies, abiraterone has demonstrated the ability to selectively inhibit the target enzyme, resulting in inhibition of testosterone production in both the adrenals and the testes.
Technical information:
| Chemical Formula: | C24H31NO | |
| CAS #: | 154229-19-3 | |
| Molecular Weight: | 349.51 | |
| Purity: | >98% | |
| Apperance: | White solid | |
| Chemical Name: | (3S,8R,9S,10R,13S,14S)-10,13-dimethyl-17-(pyridin-3-yl)-2,3,4,7,8,9,10,11,12,13,14,15- dodecahydro-1H-cyclopenta[a]phenanthren-3-ol | |
| Solubility: | Up to 50 mM in DMSO |
Shipping Condition: The product is shipped in a glass vial at ambient temperature.
Storage: For longer shelf life, store solid powder at 4oC desiccated, or store DMSO solution at -20oC.
Reference:
| 1. | Attard G et al. Selective inhibition of CYP17 with abiraterone acetate is highly active in the treatment of castration-resistant prostate cancer. J Clin Oncol. 2009;27(23):3742-8. |
| 2. | Attard G et al. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J Clin Oncol. 2008;26(28):4563-71. |
Other Information:
Product Specification (pdf)
MSDS (pdf)