Loading... Please wait...
Loading... Please wait...- Home
- Disease Area
- Lung Disease
- VX-809 (Lumacaftor)
Product Description
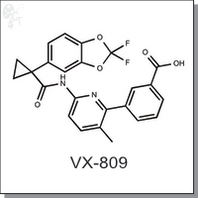
VX-809 (Lumacaftor) is a small molecular corrector and rescues folding defects of F508del mutation in cystic fibrosis transmembrane conductance regulator (CFTR). F508del is the most frequent mutation in CFTR gene that leads to cystic fibrosis (CF). VX-809 (Lumacaftor) directly binds to the pocket created by the deleted F508 in CFTR and promotes the cellular processing and maturation of F508del-CFTR in primary human bronchial epithelia (EC50 81 nM). [1] [2]
VX-809 (Lumacaftor) has been approved for cystic fibrosis treatment and also subjected to multiple Phase III clinical trials in cystic fibrosis patients. [3] [4]
Technical information:
| Chemical Formula: | C24H18F2N2O5 | |
| CAS #: | 936727-05-8 | |
| Molecular Weight: | 452.41 | |
| Purity: | > 98% | |
| Appearance: | White | |
| Chemical Name: | 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl)cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid | |
| Solubility: | Up to 100 mM in DMSO | |
| Synonyms: | VX-809, VX809, Lumacaftor |
Shipping Condition: The product is shipped in a glass vial at ambient temperature.
Storage condition: For longer shelf life, store solid powder or DMSO solution at -20oC desiccated.
Reference:
| 1. | Van Goor F, et al. Correction of the F508del-CFTR protein processing defect in vitro by the investigational drug VX-809. Proc Natl Acad Sci U S A. 2011; 108(46):18843-8 Pubmed ID: 21976485 |
| 2. | Okiyoneda T, et al. Mechanism-based corrector combination restores ?F508-CFTR folding and function. Nat Chem Biol. 2013; 9(7):444-54 Pubmed ID: 23666117 |
| 3. | Rollover Study to Evaluate the Safety and Efficacy of Long-term Treatment With Lumacaftor in Combination With Ivacaftor. clinicaltrials.gov/NCT02544451 |
| 4. | A Study to Evaluate the Efficacy and Safety of Lumacaftor in Combination With Ivacaftor in Subjects With CF, Homozygous for the F508del-CFTR Mutation. clinicaltrials.gov/NCT02514473 |
Other Information:
Product Specification (pdf)
MSDS (pdf)
Certificate of Analysis is available upon request.