Loading... Please wait...
Loading... Please wait...- Home
- Disease Area
- Immunology
- VX-702 | P38 MAPK inhibitor
- Home
- Molecular Target
- Ser/Thr Kinase
- MAPK cascade
- MAPK/P38/ERK/JNK
- VX-702 | P38 MAPK inhibitor
Product Description
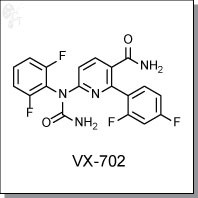
VX-702 is an orally-available, aminopyridine-based, ATP-competitive inhibitor of p38 MAPK with a Kd of 3.7 nM and 17 nM at 10 uM for p38a and p38b, respectively. [1] In an ex vivo blood assay primed with LPS< VX-702 dose-dependently inhibited the production of IL-6, IL-1b, TNFa at IC50 of 59, 122, and 99 ng/mL, respectively. [2] VX-702 was found to be equivalent to prednisolone and methotrexate in a mouse collagen-induced arthritis model.
Clinical efficacy models plus transient suppression of inflammation biomarkers suggest that p38 MAPK inhibition by agents such as VX-702 may not be a viable approach to the treatment of chronic inflammation in RA. [3]
Technical information:
| Chemical Formula: | C19H12F4N4O2 | |
| CAS #: | 479543-46-9 | |
| Molecular Weight: | 404.32 | |
| Purity: | >98% | |
| Appearance: | White | |
| Chemical Name: | 1-(5-carbamoyl-6-(2,4-difluorophenyl)pyridin-2-yl)-1-(2,6-difluorophenyl)urea | |
| Solubility: | Up to 100 mM in DMSO | |
| Synonyms: | VX-702, VX702 |
Shipping Condition: The product is shipped in a glass vial at ambient temperature.
Storage condition: For longer shelf life, store solid powder at 4oC desiccated, or store DMSO solution at -20oC.
Reference:
| 1. | Goldstein et al., Selective p38alpha inhibitors clinically evaluated for the treatment of chronic inflammatory disorders. J. Med. Chem. 2010, 53, 2345-2353. Pubmed ID: 19950901 |
| 2. | Ding, C., Drug evaluation: VX-702, a MAP kinase inhibitor for rheumatoid arthritis and acute coronary syndrome. Curr. Opin. Invest. Drugs, 2006, 7(11), 1020-1025. Pubmed ID: 17117592 |
| 3. | Damjanov et al., Efficacy, pharmacodynamics, and safety of VX-702, a novel p38 MAPK inhibitor, in rheumatoid arthritis: results of two randomized, double-blind, placebo-controlled clinical studies. Arthritis Rheum., 2009, 60(5), 1232-1241. Pubmed ID: 19404957 |
Other Information:
Product Specification (pdf)
MSDS (pdf)
Certificate of Analysis is available upon request.