Loading... Please wait...
Loading... Please wait...- Home
- Disease Area
- Infectious Disease
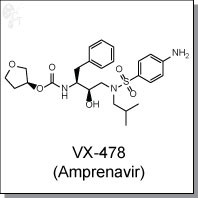
- VX-478 (Amprenavir) | HIV-1 protease inhibitor
- Home
- Molecular Target
- Protease
- Asp/Glu Protease
- VX-478 (Amprenavir) | HIV-1 protease inhibitor
Product Description
Amprenavir (Agenerase, VX-478), is an orally-available, sulfonamide-based inhibitor of HIV-1 protease, with relatively weak potency towards HIV-2, at IC50 values of 0.6 and 19 nM, respectively. [1] Similar to other HIV-1 protease inhibitors, Amprenavir binds to the active site of HIV-1 and inhibits the processing of the gag and gag-pol polyprotein precursors. The drug concentration of Amprenavir required to reduce viral production by 50% (IC50) against HIV-1 strain IIIB was 0.08 uM in acutely infected cells and 0.41 uM in chronically infected cells. Using a different cell line derived from a human T-cell lymphoma, the IC90 of Amprenavir against HIV-1 strain IIIB was 0.079 uM. [1]
Human dose-escalation studies showed that Amprenavir was well tolerated at single doses up to 1200 mg with minimal effect on absorption by food administration, unlike with other protease inhibitors such as indinavir, nelfinavir, and saquinavir. [2]
Technical information:
| Chemical Formula: | C25H35N3O6S | |
| CAS #: | 161814-49-9 | |
| Molecular Weight: | 505.63 | |
| Purity: | >98% | |
| Appearance: | White | |
| Chemical Name: | (3S)-oxolan-3-yl N-[(2S,3R)-3-hydroxy-4-[N-(2-methylpropyl)(4-aminobenzene)sulfonamido]-1-phenylbutan-2-yl]carbamate | |
| Solubility: | Up to 30 mM in DMSO | |
| Synonyms: | VX-478, VX478, Amprenavir, Agenerase |
Shipping Condition: The product is shipped in a glass vial at ambient temperature.
Storage condition: For longer shelf life, store solid powder at 4oC desiccated, or store DMSO solution at -20oC.
Reference:
| 1. | Fung et al., Amprenavir: a new human immunodeficiency virus type 1 protease inhibitor. Clin. Therapeutics, 2000, 22(5), 549-572. Pubmed ID: 10868554 |
| 2. | Sadler et al., Safety and pharmacokinetics of amprenavir (141W94), a human immunodeficiency virus (HIV) type 1 protease inhibitor, following oral administration of single doses to HIV-infected adults. Antimicrob. Angets. Chemother. 1999, 43(7), 1686-1692. Pubmed ID: 10390223 |
Other Information:
Product Specification (pdf)
MSDS (pdf)
Certificate of Analysis is available upon request.