Loading... Please wait...
Loading... Please wait...- Home
- Disease Area
- Oncology
- PF-04217903 | cMet inhibitor
- Home
- Molecular Target
- Tyrosine Kinase
- MET/RYK
- PF-04217903 | cMet inhibitor
Product Description
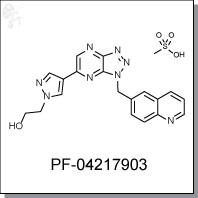
PF-04217903 is a triazolopyrazine-based, ATP-competitive inhibitor of c-Met with Ki and cellular IC50 potency of 4 nM and 5 nM, respectively. [1] In a broad panell of over 200 kinases, PF-04217903 showed >1000-fold selectivity for c-Met. For c-Met mutations, PF-04217903 exhibited activities of 6.4 nM, 6.7 nM, and 3.1 nM for endogenous c-Met-R988C, endogenous C-Met-T1010I, and entineered c-Met-H1094R, respectively. [3]
Additionally, PF-04217903 inhibited proliferation, cell survival, migration/invasion in MET-amplified cell lines in vitro and was efficacious in tumor models with either MET gene amplification or hepatocyte growth factor (HGF)/c-Met autocrine loop in vivo. [2, 4] Efficacy was dose-dependent and correlated with inhibition of c-Met phosphorylation and downstream signaling.
Due to its exquisite selectivity profile, PF-04217903 can be considered as a powerful tool in probing c-Met catalytic activity in preclinical models for cancer progression.
Technical information:
| Chemical Formula: | C20H20N8O4S | |
| CAS #: | 956906-93-7 | |
| Molecular Weight: | 468.49 | |
| Purity: | >98% | |
| Appearance: | White | |
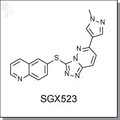
| Chemical Name: | 2-(4-(3-(quinolin-6-ylmethyl)-3H-[1,2,3]triazolo[4,5-b]pyrazin-5-yl)-1H-pyrazol-1-yl)ethanol mesylate | |
| Solubility: | Up to 15 mM in DMSO | |
| Synonyms: | PF-04217903, PF 04217903, PF04217903 |
Shipping Condition: The product is shipped in a glass vial at ambient temperature.
Storage condition: For longer shelf life, store solid powder at 4oC desiccated, or store DMSO solution at -20oC.
Reference:
| 1. | Cui et al., Discovery of a novel class of exquisitely selective mesenchymal-epithelial transition factor (c-MET) protein kinase inhibitors and identification of the clinical candidate 2-(4-(1-(quinolin-6-ylmethyl)-1H-[1,2,3]triazolo[4,5-b]pyrazin-6-yl)-1H-pyrazol-1-yl)ethanol (PF-04217903) for the treatment of cancer. J. Med. Chem. 2012, 55, 8091-8109. Pubmed ID: 22924734 |
| 2. | Zou et al., Sensitivity of selected human tumor models to PF-04217903, a novel selective c-Met kinase inhibitor. Mol. Cancer Ther. 2012, 11, 1036-1047. Pubmed ID: 22389468 |
| 3. | Timofeevski et al., Enzymatic characterization of c-Met receptor tyrosine kinase oncogenic mutants and kinetic studies with aminopyridine and triazolopyrazine inhibitors. Biochemistry, 2009, 48, 5339-5349. Pubmed ID: 19459657 |
| 4. | Shojaei et al., HGF/c-Met acts as an alternative angiogenic pathway in sunitinib-resistant tumors. Cancer Res. 2010, 70, 10090-10100. Pubmed ID: 22389468 |
Other Information:
Product Specification (pdf)
MSDS (pdf)
Certificate of Analysis is available upon request.