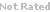 Loading... Please wait...
Loading... Please wait...Product Description
PD168393 is a first generation quinazolin amine irreversible EGFR inhibitors that alkylates Cys-773 within the ATP binding pocket [1]. While this compound did not progress into clinical application due to limited effect on EGFR mutant T790M, numerous EGFR research used this as a reference compound to explore new combinatory treatment strategies, evaluate application of EGFR inhibitors in new in vitro and in vivo cancer model systems, and compare mechanisms of action for new generations of EGFR inhibitors [2,3].
Technical information:
| Chemical Formula: | C17H13BrN4O | |
| CAS #: | 194423-15-9 | |
| Molecular Weight: | 369.22 | |
| Purity: | > 98% | |
| Appearance: | Orange | |
| Chemical Name: | N-(4-((3-bromophenyl)amino)quinazolin-6-yl)acrylamide | |
| Solubility: | Up to 200 mM in DMSO | |
| Synonyms: | PD168393 |
Shipping Condition: The product is shipped in a glass vial at ambient temperature.
Storage condition: For longer shelf life, store solid powder or DMSO solution at -20oC desiccated.
Reference:
| 1. | Fry DW, et al. Specific, irreversible inactivation of the epidermal growth factor receptor and erbB2, by a new class of tyrosine kinase inhibitor. Proc Natl Acad Sci 1998; 95(20):12022-7. Pubmed ID: 9751783 |
| 2. | Sos ML, et al. Chemogenomic Profiling Provides Insights into the Limited Activity of Irreversible EGFR Inhibitors in Tumor Cells Expressing the T790M EGFR Resistance Mutation. Cancer Res 2010; 70(3): 86874. Pubmed ID:20103621 |
| 3. | Ghosh AK, et al. Covalent Inhibition in Drug Discovery. Chem Med Chem 2019; 14(9): 889906. Pubmed ID: 30816012 |
Other Information:
Product Specification (pdf)
MSDS (pdf)
Certificate of Analysis is available upon request.