Loading... Please wait...
Loading... Please wait...- Home
- Disease Area
- Infectious Disease
- MK-0518 (Raltegravir) | HIV-1 integrase inhibitor
- Home
- Cellular Mechanism
- Metabolism
- DNA/RNA Synthesis
- MK-0518 (Raltegravir) | HIV-1 integrase inhibitor
Product Description
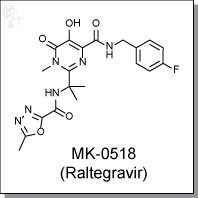
Raltegravir (Isentress)is an first-in-class, orally-available, oxadiazole-based inhibitor of HIV-1 integrase, inhibiting strand transfer in vitro with an IC50 of 2-7 nM. [1] It is >1000-fold more selective for HIV-1 integrase when compared to other phosphoryltransferases, such as the polymerase and RNase H activities of HIV-1 reverse transcriptase and the human polymerases alpha, beta, and gamma.
Raltegravir has a IC95 in human T lymphoid cell cultures of 31 nM and was also active against HIV-2 when tested in CEMx174 cells, with an IC95 of 6nM. [2]
Raltegravir has been shown to be a potent and selective against Xenotropic murine leukemia-related retrovirus (XMRV) at submicromolar concentrations in MCF-7 breast cancer (EC50 = 5 nM, EC90 = 3.5 uM) and LNCaP prostate cancer (EC50 = 30 nM, EC90 0.46 um) cell lines. [3]
Technical information:
| Chemical Formula: | C20H21FN6O5 | |
| CAS #: | 871038-72-1 | |
| Molecular Weight: | 444.42 | |
| Purity: | >98% | |
| Appearance: | White | |
| Chemical Name: | N-(4-fluorobenzyl)-5-hydroxy-1-methyl-2-(2-(2-methyl-1,3,4-oxadiazole-5-carboxamido)propan-2-yl)-6-oxo-1,6-dihydropyrimidine-4-carboxamide | |
| Solubility: | Up to 100 mM in DMSO | |
| Synonyms: | Raltegravir, MK-0518, MK0518, Isentress |
Shipping Condition: The product is shipped in a glass vial at ambient temperature.
Storage condition: For longer shelf life, store solid powder at 4oC desiccated, or store DMSO solution at -20oC.
Reference:
| 1. | Temesgen et al., Raltegravir: first in class HIV integrase inhibitor. Ther. Clin. Risk Management, 2008, 4(2), 493-500. Pubmed ID: 18728839 |
| 2. | Hicks et al., Raltegravir: the first HIV type 1 integrase inhibitor. Clin. Infect. Dis. 2009, 48(7), 931-939. Pubmed ID: 19231980 |
| 3. | Singh et al., Raltegravir is a potent inhibitor of XMRV, a virus implicated in prostate cancer and chronic fatigue syndrome. PloS ONE 2010, 4(4), e9948. Pubmed ID: 20376347 |
Other Information:
Product Specification (pdf)
MSDS (pdf)
Certificate of Analysis is available upon request.