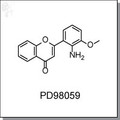
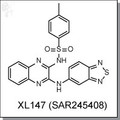
Loading... Please wait...
Loading... Please wait...- Home
- Disease Area
- Cardiovascular & Metabolic
- LY2484595 (Evacetrapib) |CETP inhibitor
- Home
- Cellular Mechanism
- Metabolism
- LY2484595 (Evacetrapib) |CETP inhibitor
Product Description
LY-2484595 (Evacetrapib) is a benzazepine-based, selective inhibitor of cholesteryl ester transfer protein (CETP) at an IC50 value of 5.5 nM for human recombinant CETP. Inhibition in human plasma was 3.6 nM IC50. [1] In H295R cells at concentrations up to 10 uM, LY-2484595 has been shown to elevate HDL cholesterol without inducing aldosterone, cortisol synthesis, or increasing blood pressure.
In a randomized controlled trial of 398 patients with elevated LDL and low HDL, LY-2484595 was shown to be effective in lowering LDL and raising HDL. In combination with statins, LY-2484595 showed greater efficacy in lowering LDL with no adverse effects. [2]
Technical information:
| Chemical Formula: | C31H36F6N6O2 | |
| CAS #: | 1186486-62-3 | |
| Molecular Weight: | 638.65 | |
| Purity: | > 98% | |
| Appearance: | White Crystalline | |
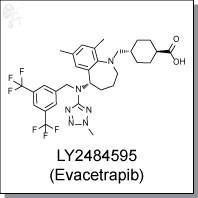
| Chemical Name: | Trans-4-({(5S)-5-[{[3,5-bis(trifluoromethyl)phenyl]methyl}(2-methyl-2H-tetrazol-5- yl)amino]-7,9-dimethyl-2,3,4,5-tetrahydro-1H-benzazepin-1-yl}methyl) cyclohexanecarboxylic acid | |
| Solubility: | Up to 22 mM in DMSO | |
| Synonyms: | LY-2484595, LY 2484595, LY2484595, Evacetrapib |
Shipping Condition: The product is shipped in a glass vial at ambient temperature.
Storage condition: For longer shelf life, store solid powder at 4oC desiccated, or store DMSO solution at -20oC.
Reference:
| 1. | Cao et al., Evacetrapib is a novel, potent, and selective inhibitor of cholesteryl ester transfer protein that elevates HDL cholesterol without inducing aldosterone or increasing blood pressure. J. Lipid Res. 2011, 52, 2169-2176. Pubmed ID: 21957197 |
| 2. | Nicholls et al., Effects of the CETP inhibitor evacetrapib administered as monotherapy or in combination with statins on HDL and LDL cholesterol: a randomized controlled trial. JAMA 2011, 306(19), 2099-2109. Pubmed ID: 22089718 |
Other Information:
Product Specification (pdf)
MSDS (pdf)
Certificate of Analysis is available upon request.