Loading... Please wait...
Loading... Please wait...- Home
- Signaling Pathway
- PI3k/Akt/mTOR
- GSK2126458 | PI3K/mTOR inhibitor
- Home
- Disease Area
- Oncology
- GSK2126458 | PI3K/mTOR inhibitor
Product Description
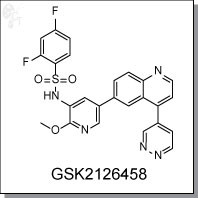
GSK2126458 is an orally available, pyridazine-quinoline-based inhibitor of of p110?, p110?, p110?, p110?, mTORC1, and mTORC2 with Ki of 0.019 nM, 0.13 nM, 0.024 nM, 0.06 nM, 0.18 nM and 0.3 nM, respectively. [1] In mechanistic assays, GSK2126458 induced significant reduction in levels of pAKT-S473 (0.41 nM in T47D and 0.18 nM in BT474). It also inhibits phosphorylation of AKT-T308 and p70S6K at low nanomolar concentrations. Induction of caspase 3 and 7 activity suggests that GSK2126458 utilizes apoptosis as a mechanism for cell death. [2]
Combination of GSK2126458 with B-Raf inhibitor GSK2118436 (Dabrafenib) enhanced cell growth inhibition and decreased S6 ribosomal protein phosphorylation in NRAS and MEK mutant clones. Combination of GSK2126458 and MEK inhibitor GSK1120212 (trametinib) has been studied in Phase I clinical trials. [3]
Technical information:
| Chemical Formula: | C25H17F2N5O3S | |
| CAS #: | 1086062-66-9 | |
| Molecular Weight: | 505.5 | |
| Purity: | > 98% | |
| Appearance: | White | |
| Chemical Name: | 2,4-difluoro-N-(2-methoxy-5-(4-(pyridazin-4-yl)quinolin-6-yl)pyridin-3-yl)benzenesulfonamide | |
| Solubility: | Up to 100mM in DMSO | |
| Synonyms: | GSK-2126458, GSK2126458, GSK458 |
Shipping Condition: The product is shipped in a glass vial at ambient temperature.
Storage condition: For longer shelf life, store solid powder at 4oC desiccated, or store DMSO solution at -20oC.
Reference:
| 1. | Knight et al., ACS Med. Chem. Lett. 2010, 1, 39-43. |
| 2. | Hardwick et al., Mol. Cancer Ther. 2009, 8(12), Supplement I, Abstract C63. |
| 3. | Greger et al., Combinations of BRAF, MEK, and PI3K/mTOR inhibitors overcome acquired resistance to the BRAF inhibitor GSK2118436 dabrafenib, mediated by NRAS or MEK mutations. Mol. Cancer Ther. 2012, 11(4), 909-920. Pubmed ID: 22389471 |
Other Information:
Product Specification (pdf)
MSDS (pdf)
Certificate of Analysis is available upon request.