Loading... Please wait...
Loading... Please wait...- Home
- Disease Area
- Oncology
- Fulvestrant (ICI-182780)
Product Description
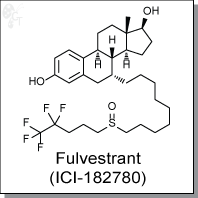
Fulvestrant (ICI-182780) is an estrogen receptor (ER) antagonist with an IC50 of 9.4 nM in a cell-free assay. It shows potent inhibitory effect in the growth of MCF-7 human breast cancer cells, with an IC50 of 0.29 nM. [1] Unlike tamoxifen, Fulvestrant (ICI-182780) is devoid of agonist activity and serves as a ??pure?? antioestrogen. [1]
Binding of Fulvestrant to the ER induces a rapid loss of ER protein from breast carcinoma cells. [2]
Fulvestrant (ICI-182780) is approved by FDA for the treatment of ER?-positive metastatic breast cancer in postmenopausal women with disease progression following anti-estrogen therapy. It has been also subjected in a Phase III clinical trial in patients with HER2 negative postmenopausal metastatic breast cancer. [3]"
Technical information:
| Chemical Formula: | C32H47F5O3S | |
| CAS #: | 129453-61-8 | |
| Molecular Weight: | 606.77 | |
| Purity: | > 98% | |
| Appearance: | White | |
| Chemical Name: | 7-[9-[(4,4,5,5,5-pentafluoropentyl)sulfinyl]nonyl]-(7?,17?)-estra-1,3,5(10)-triene-3,17-diol | |
| Solubility: | Up to 100 mM in DMSO | |
| Synonyms: | Fulvestrant, ICI-182780, ZD 9238, Faslodex |
Shipping Condition: The product is shipped in a glass vial at ambient temperature.
Storage condition: For longer shelf life, store solid powder or DMSO solution at -20oC desiccated.
Reference:
| 1. | Wakeling AE, et al. A potent specific pure antiestrogen with clinical potential. Cancer Res. 1991; 51(15):3867-73 Pubmed ID: 1855205 |
| 2. | Wakeling AE. Similarities and distinctions in the mode of action of different classes of antioestrogens. Endocr Relat Cancer. 2000; 7(1):17-28 Pubmed ID: 10808193 |
| 3. | Fulvestrant as Maintenance Therapy After First-line Chemotherapy in HER2 - Postmenopausal MBC Patients (FUMANCE). clinicaltrials/NCT02383030 |
Other Information:
Product Specification (pdf)
MSDS (pdf)
Certificate of Analysis is available upon request.