Loading... Please wait...
Loading... Please wait...- Home
- Disease Area
- Cardiovascular & Metabolic
- BMS-512148 (Dapagliflozin) | SGLT2 inhibitor
- Home
- Cellular Mechanism
- Metabolism
- BMS-512148 (Dapagliflozin) | SGLT2 inhibitor
Product Description
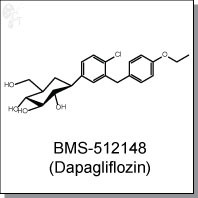
Dapagliflozin (BMS-512148) is an orally-available C-aryl glucoside diphenylmethanol inhibitor of SGLT2 (IC50 1.1 nM) for the treatment of Type 1 and Type 2 diabetes. It is the first approved SGLT2 inhibitor (European Union as Forxiga) for the treatment of diabetes. Its excellent selectivity over SGLT1 (IC50 1390 nM) ensures that it does not interfere with intestinal glucose absorption. Dapagliflozin minimally inhibits glucose transporters GLUT1 and GLUT2 and modestly inhibits GLUT4. [1]
Dapagliflozin removes excess glucose and its associated calories in urine, which in turn reduces blood sugar levels. Clinical studies have shown concurrent reductions in weight and blood pressure. [2] In combination with metformin, the weight loss was statistically significant, dose-dependent, and persisted for over two years. [3]
Technical information:
| Chemical Formula: | C21H25ClO6 | |
| CAS #: | 461432-26-8 | |
| Molecular Weight: | 408.87 | |
| Purity: | >98% | |
| Appearance: | White | |
| Chemical Name: | (2S,3R,4R,5S,6R)-2-(3-(4-ethoxybenzyl)-4-chlorophenyl)-6-(hydroxymethyl)-tetrahydro-2H-pyran-3,4,5-triol | |
| Solubility: | Up to 100 mM in DMSO | |
| Synonyms: | BMS-512148, BMS 512148, BMS512148, Dapagliflozin |
Shipping Condition: The product is shipped in a glass vial at ambient temperature.
Storage condition: For longer shelf life, store solid powder at 4oC desiccated, or store DMSO solution at -20oC.
Reference:
| 1. | Shah et al., Dapagliflozin: a novel sodium-glucose cotransporter type 2 inhibitor for the treatment of type 2 diabetes mellitus. Pharmacotherapy 2012, 32(1), 80-94. Pubmed ID: 22392830 |
| 2. | AstraZeneca website: http://www.astrazeneca.com/Media/Press-releases/Article/20121114--forxiga-eu-approval-type-2-diabetes |
| 3. | Chao et al., Dapagliflozin: an evidence-based review of its potential in the treatment of type-2 diabetes. Core Evidence 2012, 7, 21-28. Pubmed ID: 22701099 |
Other Information:
Product Specification (pdf)
MSDS (pdf)
Certificate of Analysis is available upon request.