Loading... Please wait...
Loading... Please wait...- Home
- Disease Area
- Oncology
- Axitinib (AG 013736)
- Home
- Molecular Target
- Tyrosine Kinase
- VEGFR Family
- Axitinib (AG 013736)
Product Description
Axitinib (AG 013736) is a potent multi-target tyrosine kinase inhibitor (TKI), which inhibits VEGFR1, VEGFR2, VEGFR3, PDGFR?, PDGFR? and c-Kit with IC50 values of 1.2 nM, 0.2 nM, 0.1 nM-0.3 nM, 1.6 nM, 5.0 nM, and 1.7 nM, respectively. [1] In human xenograft tumor models (nude mice), Axitinib (AG 013736) shows consistent antitumor efficacy across various tumor types, which is associated with reduction in vascular angiogenesis and tumor proliferation and increase in tumor apoptosis. [1]
Axitinib (AG 013736) resulted in significantly longer progression-free survival compared with sorafenib and has been approved as a second-line therapy of advanced renal cell carcinoma. [2]
Technical information:
| Chemical Formula: | C22H18N4OS | |
| CAS #: | 319460-85-0 | |
| Molecular Weight: | 386.47 | |
| Purity: | > 98% | |
| Appearance: | White | |
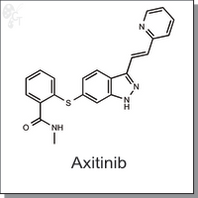
| Chemical Name: | N-methyl-2-[[3-[(1E)-2-(2-pyridinyl)ethenyl]-1H-indazol-6-yl]thio]-benzamide | |
| Solubility: | Up to 100 mM in DMSO | |
| Synonyms: | Axitinib, AG 013736 |
Shipping Condition: The product is shipped in a glass vial at ambient temperature.
Storage condition: For longer shelf life, store solid powder or DMSO solution at -20oC desiccated.
Reference:
| 1. | Hu-Lowe DD, et al. Nonclinical antiangiogenesis and antitumor activities of axitinib (AG-013736), an oral, potent, and selective inhibitor of vascular endothelial growth factor receptor tyrosine kinases 1, 2, 3. Clin Cancer Res. 2008; 14(22):7272-83 Pubmed ID: 19010843 |
| 2. | Axitinib in Metastatic Renal Cell Carcinoma Patients With Favorable Prognostic Factors (FavorAx). clinicaltrials.gov/NCT02700568 |
Other Information:
Product Specification (pdf)
MSDS (pdf)
Certificate of Analysis is available upon request.