 Loading... Please wait...
Loading... Please wait...- Home
- Disease Area
- Oncology
- Temozolomide | DNA alkylating agent
- Home
- Cellular Mechanism
- DNA Damage & Repair
- Temozolomide | DNA alkylating agent
Product Description
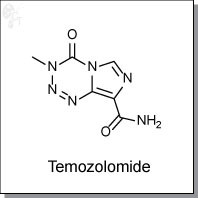
Temozolomide is an highly orally-available, imidazotetrazine-based, monofunctional DNA alkylating agent. In vivo it spontaneously degrades and reacts with water to release a methyldiazonium cation which preferentially methylates DNA at N7 positions of guanine in guanine rich regions. The active agent also methylates N3 adenine and O6 guanine. [1]
Temozolomide, which enters the cerebrospinal fluid and does not require hepatic metabolism, demonstrates schedule-dependent antitumor activity against highly-resistant gliomas. [2] In Phase 1 clinical studies, temozolomide showed 100% p.o. bioavailability.
In L-1210 leukemia cells, temozolomide caused a dose-dependent accumulation of cells in the SL-G2-M phase. [3]
Technical information:
| Chemical Formula: | C6H6N6O2 | |
| CAS #: | 85622-93-1 | |
| Molecular Weight: | 194.15 | |
| Purity: | >99% | |
| Appearance: | White | |
| Chemical Name: | 4-methyl-5-oxo- 2,3,4,6,8-pentazabicyclo [4.3.0] nona-2,7,9-triene- 9-carboxamide | |
| Solubility: | Up to 100 mM in DMSO | |
| Synonyms: | Temozolomide, Temodar, Methazolastone |
Shipping Condition: The product is shipped in a glass vial at ambient temperature.
Storage condition: For longer shelf life, store solid powder at 4oC desiccated, or store DMSO solution at -20oC.
Reference:
| 1. | Zhang et al., Temozolomide: mechanisms of action, repair and resistance. Curr. Mol. Pharmacol. 2012, 5, 102-114. Pubmed ID: 22122467 |
| 2. | Friedman et al., Temozolomide and treatment of malignant glioma. Clin. Cancer Res. 2000, 6, 2585-2597. Pubmed ID: 10914698 |
| 3. | Catapano et al., In vitro and in vivo methazolastone-induced DNA damage and repair in L-1210 leukemia sensitive and resistant to chloroethylnitrosoureas. Cancer Res. 1987, 47, 4884-4889. Pubmed ID: 3621181 |
Other Information:
Product Specification (pdf)
MSDS (pdf)
Certificate of Analysis is available upon request.